Research gives new insight into how signals are transmitted between cells
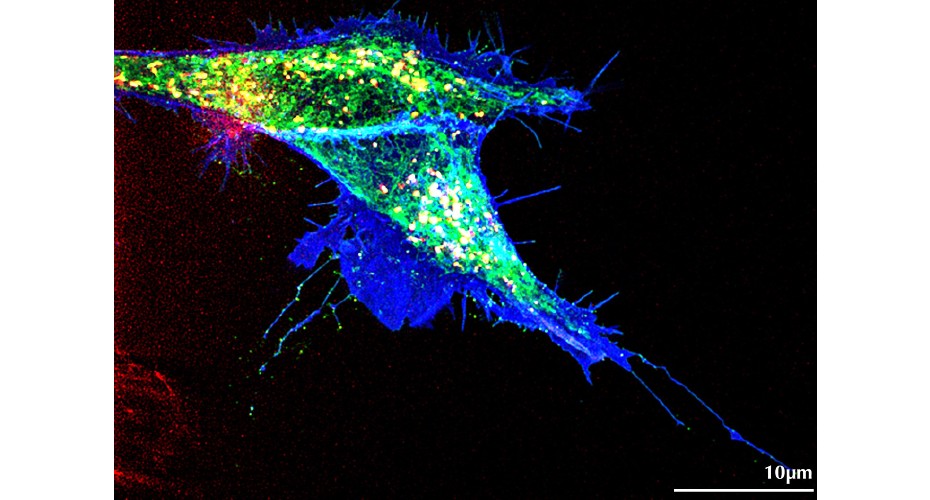
The image shows in vivo super-resolution analysis taken with a Lattice-Structured Illumination Microscope
The quest to gain a greater and more in-depth understanding about how signals are transmitted between cells has taken a remarkable step forward.
New research, led by experts from the University of Exeter’s Living Systems Institute, has discovered a new synergy between transport and signalling within cells for the first time.
In all multicellular organisms, cells ‘communicate’ with each other in order to be able to grow and function properly.
In order to conduct this communication, cells express signal molecules and hand them over to the neighbouring cells which provide the exact docking molecules – similar to a key being placed into a lock – which in turn activates a cascade of responses in the cell.
One of the most essential messaging systems is the Wnt signalling network, which acts as the key to fit a lock called Ror2 to change the movement of these cells.
In the new research, scientists used special techniques to mark Wnt5b and Ror2 fluorescently in developing zebrafish embryos.
Then, using state-of-the-art high-resolution microscopy from the Bioimaging Centre, they were able see how these components move around in real time.
They found that the Wnt5b and Ror2 were made by the very same cells, and travel together from one cell to another using tiny cellular extensions called cytonemes. The research team discovered the ‘key and lock’ are connected throughout the journey to the new cell, rather than travel separately.
The research challenges conventional understandings of how cells respond to signals, and suggests that even cells without the right lock can react if they get a functional key-lock duo through these thin cytonemes.
The experts now want to explore how these tiny threads form, how the key-lock pairs are carried along these cell threads to the correct cells, and if this also happens in other critical signalling systems in the body.
The next phase of the research could help advance understanding of illnesses like cancer, where this signalling process goes wrong, and develop new strategies to combat these devastating disorders.
Professor Steffen Scholpp, lead author of the study and an expert in Cell and Developmental Biology at the LSI, said: “As cell signalling underpins multicellular life, our research findings will be transformative for the biosciences. Based on these results, we must redefine our strategies for controlling signalling. Cytonemes can now be targeted for designing new therapeutics for many diseases caused by defective cell communication.”
Cytoneme-mediated transport of active Wnt5b/Ror2 complexes in zebrafish is published in the journal Nature.



