Ultra-strong ‘threads’ made of proteins help tiny organisms live in boiling acid
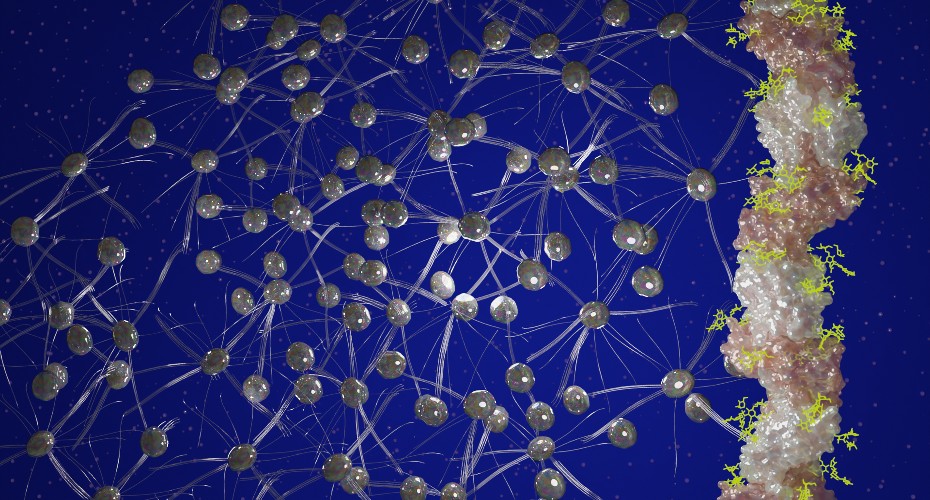
Scientists have discovered a new chain-like structure that helps single-celled organisms survive in the sulfur-rich hot acid springs of Yellowstone National Park in the USA.
While Yellowstone’s hot springs are famous landmarks, the fact that life can survive in their boiling and highly acidic waters is equally stunning.
To discover how this is possible, an international research team – led by the University of Exeter – studied an acid-loving species of archaea called Sulfolobus acidocaldarius.
Using a cutting-edge imaging technique called electron cryo-microscopy, they examined the hair-like protein filaments produced by the species – and found a previously unknown protein that forms extremely stable chain-like fibres.
“The hot springs in Yellowstone are so corrosive that only very special life forms can survive within them ” said Dr Bertram Daum, of Exeter’s Living Systems Institute and senior author of the study.
“Of all life on Earth, archaeal species are most commonly found in such extreme conditions, and S. acidocaldarius is a tough organism that thrives at around 80°C and very acidic pH values.
“Through the oxidation of sulphur within the springs it calls home, S. acidocaldarius even creates the toxic sulfuric acid it inhabits.”
The team set out to understand how S. acidocaldarius cells come together to form interconnected communities on surfaces, called biofilms.
S. acidocaldarius produces four different protein filaments, each highly stable and with a unique function – and understanding the structure of these could not only reveal how this species survives, but could aid the development of strong, yet biodegradable nanomaterials.
“We examined the structure of one of these filaments, called ‘thread’,” Dr Daum said.
“Our collaborators in Germany grew S. acidocaldarius in special incubators and isolated the threads from the cells.
“We then froze the threads at very low temperatures and imaged them using a transmission electron microscope.
“Using sophisticated image analysis software, we generated a highly detailed three-dimensional image of the thread, which allowed us to visualise it at atomic resolution.”
To the team’s surprise, this revealed a previously unseen class of archaeal protein filaments.
“The threads are made of tadpole-shaped protein subunits, which are concatenated like beads on a string,” Mr Gaines, co-author of the study explained.
“The subunits are held together by extremely strong links; each tadpole-shaped subunit inserts its tail the head of the next subunit along the chain.”
These strong bonds allow the cells to join and stay connected in biofilms, even under the most adverse conditions.
The study, published in the journal Nature Communications, is entitled: “Electron cryo-microscopy reveals the structure of the archaeal thread filament.”



